Fission of uranium nuclei. Chain reaction. Uranium nuclear fission and chain reaction
Nuclear fission is a process in which 2 (sometimes 3) fragment nuclei are formed from one atomic nucleus, which are similar in mass.
This process is beneficial for everyone β -stable nuclei with mass number A > 100.
Uranium nuclear fission was discovered in 1939 by Hahn and Strassman, who unequivocally proved that when neutrons bombard uranium nuclei U Radioactive nuclei are formed with masses and charges approximately 2 times less than the mass and charge of the uranium nucleus. In the same year, L. Meitner and O. Frischer introduced the term “ nuclear fission"and it was noted that during this process enormous energy is released, and F. Joliot-Curie and E. Fermi simultaneously found out that several neutrons are emitted during fission (fission neutrons). This became the basis for putting forward the idea self-sustaining fission chain reaction and the use of nuclear fission as a source of energy. The basis of modern nuclear energy is nuclear fission 235 U And 239 Pu under the influence of neutrons.
Nuclear fission can occur due to the fact that the rest mass of the heavy nucleus is greater than the sum of the rest masses of the fragments that arise during the fission process.
The graph shows that this process turns out to be beneficial from an energy point of view.
The mechanism of nuclear fission can be explained on the basis of the droplet model, according to which a bunch of nucleons resembles a droplet of a charged liquid. The nucleus is kept from decay by nuclear attractive forces, greater than the Coulomb repulsion forces that act between protons and tend to tear the nucleus apart.
Core 235 U has the shape of a ball. After absorbing a neutron, it is excited and deformed, acquiring an elongated shape (in the figure b), and stretches until the repulsive forces between the halves of the elongated core become greater than the attractive forces acting in the isthmus (in the figure V). After this, the nucleus breaks into two parts (in the figure G). The fragments, under the influence of Coulomb repulsive forces, fly away at a speed equal to 1/30 of the speed of light.
Emission of neutrons during fission, which we talked about above, is explained by the fact that the relative number of neutrons (relative to the number of protons) in the nucleus increases with increasing atomic number, and for the fragments formed during fission, the number of neutrons becomes greater than is possible for the nuclei of atoms with smaller numbers.
Division often occurs into fragments of unequal mass. These fragments are radioactive. After the series β -decays ultimately produce stable ions.
Except forced, it happens spontaneous fission of uranium nuclei, which was discovered in 1940 by Soviet physicists G.N. Flerov and K.A. Petrzhak. The half-life for spontaneous fission corresponds to 10 16 years, which is 2 million times greater than the half-life for α -decay of uranium.
The synthesis of nuclei occurs in thermonuclear reactions. Thermonuclear reactions is a fusion reaction of light nuclei at very high temperatures. The energy that is released during fusion (synthesis) will be maximum during the synthesis of light elements that have the lowest binding energy. When two light nuclei, such as deuterium and tritium, combine, a heavier helium nucleus with higher binding energy is formed:
With this process of nuclear fusion, significant energy is released (17.6 MeV), equal to the difference in the binding energies of a heavy nucleus and two light nuclei ![]() . The neutron produced during reactions acquires 70% of this energy. A comparison of the energy per nucleon in the reactions of nuclear fission (0.9 MeV) and fusion (17.6 MeV) shows that the fusion reaction of light nuclei is energetically more favorable than the fission reaction of heavy ones.
. The neutron produced during reactions acquires 70% of this energy. A comparison of the energy per nucleon in the reactions of nuclear fission (0.9 MeV) and fusion (17.6 MeV) shows that the fusion reaction of light nuclei is energetically more favorable than the fission reaction of heavy ones.
The fusion of nuclei occurs under the influence of nuclear attraction forces, so they must approach to distances less than 10 -14 at which nuclear forces act. This approach is prevented by the Coulomb repulsion of positively charged nuclei. It can be overcome only due to the high kinetic energy of the nuclei, which exceeds the energy of their Coulomb repulsion. From the corresponding calculations it is clear that the kinetic energy of nuclei, which is needed for the fusion reaction, can be achieved at temperatures of the order of hundreds of millions of degrees, therefore these reactions are called thermonuclear.
Thermonuclear fusion- a reaction in which, at high temperatures above 10 7 K, heavier nuclei are synthesized from light nuclei.
Thermonuclear fusion is the source of energy for all stars, including the Sun.
The main process by which thermonuclear energy is released in stars is the conversion of hydrogen into helium. Due to the mass defect in this reaction, the mass of the Sun decreases by 4 million tons every second.
The large kinetic energy that is needed for thermonuclear fusion is obtained by hydrogen nuclei as a result of strong gravitational attraction to the center of the star. After this, the fusion of helium nuclei produces heavier elements.
Thermonuclear reactions play one of the main roles in the evolution of the chemical composition of matter in the Universe. All these reactions occur with the release of energy, which is emitted by stars in the form of light over billions of years.
The implementation of controlled thermonuclear fusion would provide humanity with a new, practically inexhaustible source of energy. Both deuterium and tritium needed for its implementation are quite accessible. The first is contained in the water of the seas and oceans (in quantities sufficient for use for a million years), the second can be obtained in a nuclear reactor by irradiating liquid lithium (the reserves of which are huge) with neutrons:
One of the most important advantages of controlled thermonuclear fusion is the absence of radioactive waste during its implementation (unlike fission reactions of heavy uranium nuclei).
The main obstacle to the implementation of controlled thermonuclear fusion is the impossibility of confining high-temperature plasma using strong magnetic fields for 0.1-1. However, there is confidence that sooner or later thermonuclear reactors will be created.
So far it has only been possible to produce uncontrollable reaction explosive type synthesis in a hydrogen bomb.
Physics lesson in 9th grade
“Fission of uranium nuclei. Chain reaction"
The purpose of the lesson: To familiarize students with the process of fission of uranium atomic nuclei and the mechanism of the chain reaction.
Tasks:
educational:
study the mechanism of fission of uranium-235 nuclei; introduce the concept of critical mass; determine the factors that determine the occurrence of a chain reaction.
educational:
lead students to understand the significance of scientific discoveries and the the danger that can come from scientific achievements with a thoughtless, illiterate or immoral attitude towards them.
developing:
development of logical thinking; development of monologue and dialogic speech; development of mental operations in students: analysis, comparison, learning. Formation of an idea of the integrity of the picture of the world
Lesson type: lesson in learning new knowledge.
Competencies that the lesson aims to develop:
value-semantic - the ability to see and understand the world around us,
general cultural - the student’s mastery of the scientific picture of the world,
educational and cognitive - the ability to distinguish facts from speculation,
Communication skills - group work skills, mastery of various social roles in a team,
competencies of personal self-improvement - culture of thinking and behavior
Lesson progress: 1. Organizational moment.
A new lesson has arrived. I will smile at you, and you will smile at each other. And you will think: how good it is that we are all here together today. We are modest and kind, friendly and affectionate. We are all healthy. - Take a deep breath and exhale. Exhale yesterday's resentment, anger and anxiety. I wish us all a good lesson .
2. Checking homework.
Test.
1. What charge does the nucleus have?
1) positive 2) negative 3) the nucleus has no charge
2. What is an alpha particle?
1) electron 2) nucleus helium atom
3) electromagnetic radiation
3. How many protons and neutrons does the nucleus of a berylliumBe atom contain?
1) Z =9, N =4 2) Z =5, N =4 3) Z =4, N =5
4. The nucleus of which chemical element is formed during the α - decay of radium?
Ra → ? +He.
1) radon 2) uranium 3) fermium
5. The mass of a nucleus is always ... the sum of the masses of the nucleons of which it consists.
1) greater than 2) equal to 3) less
6. A neutron is a particle
1) having charge +1, atomic mass 1;
2) having a charge – 1, atomic mass 0;
3) having charge 0, atomic mass 1.
7.Indicate the second product of the nuclear reaction
Answers: Option 1. 1)1; 2)2; 3)3; 4)1; 5)3; 6)3; 7)3.
8. How do protons in the nucleus interact with each other electrically?
9. What is a mass defect? Write down the formula.
10. What is binding energy? Write down the formula.
Learning new material.
We recently learned that some chemical elements transform into other chemical elements during radioactive decay. What do you think will happen if you send some particle into the nucleus of an atom of some chemical element, for example, a neutron into the nucleus of uranium?
In 1939, German scientists Otto Hahn and Fritz Strassmann discovered the fission of uranium nuclei. They found that when uranium is bombarded with neutrons, elements of the middle part of the periodic table appear - radioactive isotopes of barium (Z = 56), krypton (Z = 36), etc.
Let us consider in more detail the process of fission of a uranium nucleus during bombardment with a neutron according to the figure. A neutron entering a uranium nucleus is absorbed by it. The core gets excited and begins to deform like a liquid drop.
The nucleus becomes excited and begins to deform. Why does the nucleus break into two parts? Under what forces does the rupture occur?
What forces act inside the nucleus?
– Electrostatic and nuclear.
Okay, but how do electrostatic forces manifest themselves?
– Electrostatic forces act between charged particles. The charged particle in the nucleus is the proton. Since the proton is positively charged, it means that repulsive forces act between them.
True, but how do nuclear forces manifest themselves?
– Nuclear forces are the forces of attraction between all nucleons.
So, under the influence of what forces does the nucleus rupture?
– (If difficulties arise, I ask guiding questions and lead students to the correct conclusion) Under the influence of electrostatic repulsive forces, the nucleus breaks into two parts, which fly apart in different directions and emit 2-3 neutrons.
It stretches until the electrical repulsive forces begin to prevail over the nuclear ones. The nucleus breaks into two fragments, releasing two or three neutrons. This is the technology of fission of a uranium nucleus.
The fragments fly away at very high speed. It turns out that part of the internal energy of the nucleus is converted into the kinetic energy of flying fragments and particles. The fragments end up in the environment. What do you think is happening to them?
– The fragments are slowed down in the environment.
In order not to violate the law of conservation of energy, we must say what will happen to the kinetic energy?
– The kinetic energy of the fragments is converted into internal energy of the environment.
Can you notice that the internal energy of the medium has changed?
– Yes, the environment is heating up.
Will the change in internal energy be influenced by the fact that different numbers of uranium nuclei will participate in fission?
– Of course, with the simultaneous fission of a large number of uranium nuclei, the internal energy of the environment surrounding the uranium increases.
From your chemistry course, you know that reactions can occur both with the absorption of energy and the release. What can we say about the course of the fission reaction of uranium nuclei?
– The fission reaction of uranium nuclei releases energy into the environment.
(Slide 13)
Uranium occurs in nature in the form of two isotopes: U (99.3%) and U (0.7%). In this case, the fission reaction of U occurs most intensively with slow neutrons, while U nuclei simply absorb a neutron, and fission does not occur. Therefore, the main interest is in the fission reaction of the U nucleus. Currently, about 100 different isotopes with mass numbers from about 90 to 145 are known that arise during the fission of this nucleus. Two typical fission reactions of this nucleus are:

Let us note that the energy released during the fission of uranium nuclei is enormous. For example, the complete fission of all nuclei contained in 1 kg of uranium releases the same energy as the combustion of 3000 tons of coal. Moreover, this energy can be released instantly.
(Slide 14)
We found out what will happen to the fragments, how will neutrons behave?
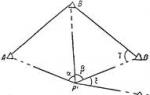
When a uranium-235 nucleus fissions, which is caused by a collision with a neutron, 2 or 3 neutrons are released. Under favorable conditions, these neutrons can hit other uranium nuclei and cause them to fission. At this stage, from 4 to 9 neutrons will appear, capable of causing new decays of uranium nuclei, etc. Such an avalanche-like process is called chain reaction. (Write in notebook: Nuclear chain reaction- a sequence of nuclear reactions, each of which is caused by a particle that appeared as a reaction product at the previous step of the sequence). We will consider the development diagram of the chain reaction of fission of uranium nuclei in more detail using a video fragment in slow motion for a more detailed consideration
We see that the total number of free neutrons in a piece of uranium increases like an avalanche over time. What could this lead to?
- To the explosion.
Why?
– The number of nuclear fissions increases and, accordingly, the energy released per unit time.
But another option is also possible, in which the number of free neutrons decreases with time, and the neutron does not meet the nucleus on its way. In this case what will happen to the chain reaction?
- It will stop.
Is it possible to use the energy of such reactions for peaceful purposes?
How should the reaction proceed?
– The reaction must proceed in such a way that the number of neutrons remains constant over time.
How can we ensure that the number of neutrons remains constant all the time?
– (guys' suggestions)
To solve this problem, you need to know what factors influence the increase and decrease in the total number of free neutrons in a piece of uranium in which a chain reaction occurs.
(Slide 15)
One of these factors is mass of uranium . The fact is that not every neutron emitted during nuclear fission causes the fission of other nuclei. If the mass (and, accordingly, the dimensions) of a piece of uranium is too small, then many neutrons will fly out of it, not having time to meet the nucleus on their way, causing its fission and thus generating a new generation of neutrons necessary to continue the reaction. In this case, the chain reaction will stop. In order for the reaction to continue, it is necessary to increase the mass of uranium to a certain value, called critical.
Why does a chain reaction become possible as mass increases?
For a chain reaction to occur, it is necessary that the so-called reproduction rate neutrons were more than one. In other words, in each subsequent generation there should be more neutrons than in the previous one. The multiplication coefficient is determined not only by the number of neutrons produced in each elementary act, but also by the conditions under which the reaction occurs - some of the neutrons can be absorbed by other nuclei or leave the reaction zone. Neutrons released during the fission of uranium-235 nuclei are capable of causing the fission of only the nuclei of the same uranium, which accounts for only 0.7% of natural uranium. This concentration is insufficient to start a chain reaction. The U isotope can also absorb neutrons, but this does not cause a chain reaction.
( Write in your notebook: Neutron multiplication factork - the ratio of the number of neutrons of the subsequent generation to the number in the previous generation in the entire volume of the neutron-multiplying medium)
A chain reaction in uranium with a high content of uranium-235 can develop only when the mass of uranium exceeds the so-called critical mass. In small pieces of uranium, most neutrons fly out without hitting any nucleus. For pure uranium-235, the critical mass is about 50 kg.
( Write in your notebook: Critical mass- the minimum amount of fissile material required to start a self-sustaining fission chain reaction).
(Slide 16)
The critical mass of uranium can be reduced many times by using so-called neutron moderators. The fact is that neutrons produced during the decay of uranium nuclei have too high speeds, and the probability of capturing slow neutrons by uranium-235 nuclei is hundreds of times greater than fast ones. The best neutron moderator is heavy water H 2 O. When interacting with neutrons, ordinary water itself turns into heavy water.
Graphite, whose nuclei do not absorb neutrons, is also a good moderator. During elastic interaction with deuterium or carbon nuclei, neutrons slow down their movement.
The use of neutron moderators and a special beryllium shell, which reflects neutrons, makes it possible to reduce the critical mass to 250 g (0.25 kg).
Write in your notebook:
Critical mass can be reduced if:
Use moderators (graphite, ordinary and heavy water)
Reflective shell (beryllium)).
And in atomic bombs, an uncontrolled nuclear chain reaction occurs when two pieces of uranium-235 quickly combine, each of which has a mass slightly below critical.
The atomic bomb is a terrible weapon. The damaging factors of which are: 1) Light radiation (including X-ray and thermal radiation); 2) Shock wave; 3) radiation contamination of the area. But the fission of uranium nuclei is also used for peaceful purposes - in nuclear reactors at nuclear power plants. We will consider the processes occurring in these cases in the next lesson.
The middle of the 20th century is defined by the acceleration of science: fantastic acceleration, the introduction of scientific achievements into production and into our lives. All this makes us think - what will science give us tomorrow?
To alleviate all the burdens of human existence is the main goal of truly progressive science. To make humanity happier - not just one, not two, but humanity. And this is very important, because, as you know, science can also act against a person. The atomic explosion in the Japanese cities of Hiroshima and Nagasaki is a tragic example of this.
So, 1945, August. The Second World War is coming to an end.
(Slide 2)
On August 6 at 1:45 a.m., an American B-29 bomber under the command of Colonel Paul Tibbetts took off from the island, which was approximately 6 hours flight time from Hiroshima.
(Slide 3)
Hiroshima after the atomic explosion.
Whose shadow wanders there unseen,
Did you go blind from trouble?
This is Hiroshima crying
In clouds of ash.
Whose voice is there in the hot darkness?
Can you hear the frenzy?
It's Nagasaki crying
On a burnt land
In this crying and sobbing
There is no falsehood
The whole world froze in anticipation -
Who will cry next?
(Slide 4)
The number of deaths from the direct impact of the explosion ranged from 70 to 80 thousand people. By the end of 1945, due to radioactive contamination and other post-effects of the explosion, the total number of deaths ranged from 90 to 166 thousand people. After 5 years, the total number of deaths reached 200,000 people.
(Slide 5)
On August 6, after receiving news of the successful atomic bombing of Hiroshima, US President Truman announced that
“We are now ready to destroy, even faster and more completely than before, all land-based production facilities of the Japanese in any city. We will destroy their docks, their factories, and their communications. Let there be no misunderstanding - we will completely destroy Japan's ability to wage war."
(Slide 6)
At 2:47 on August 9, an American B-29 bomber under the command of a major, carrying an atomic bomb on board, took off from the island. At 10:56 B-29 arrived at Nagasaki. The explosion occurred at 11:02 local time.
(Slide 7)
The number of deaths by the end of 1945 ranged from 60 to 80 thousand people. After 5 years, the total death toll, including deaths from cancer and other long-term effects of the explosion, may have reached or even exceeded 140,000.
This is the story, sad and warning
Every person is not an island,
every person is part of a large continent.
And never ask for whom the bell tolls.
He's calling for you...
Consolidation.
What did we learn about in class today? (with a mechanism of fission of uranium nuclei, with a chain reaction)
What are the conditions for a chain reaction to occur?
What is critical mass?
What is the reproduction rate?
What serves as a neutron moderator?
Reflection.
How do you feel when you leave class?
Assessment.
Homework: paragraphs 74,75, questions pp. 252-253
Uranium nuclei fission occurs in the following way: First, a neutron hits the nucleus, like a bullet hitting an apple. In the case of an apple, a bullet would either make a hole in it or blow it into pieces. When a neutron enters the nucleus, it is captured by nuclear forces. The neutron is known to be neutral, so it is not repelled by electrostatic forces.
How does a uranium nucleus fission occur?
So, having entered the nucleus, the neutron disturbs the equilibrium, and the nucleus is excited. It stretches out to the sides like a dumbbell or an infinity sign: ∞ . Nuclear forces, as is known, act at a distance commensurate with the size of the particles. When the nucleus is stretched, the effect of nuclear forces becomes insignificant for the outer particles of the “dumbbell,” while electrical forces act very powerfully at such a distance, and the nucleus is simply torn into two parts. In this case, two or three more neutrons are emitted.
Fragments of the nucleus and released neutrons scatter at great speed in different directions. The fragments are slowed down quite quickly by the environment, but their kinetic energy is enormous. It is converted into internal energy of the environment, which heats up. In this case, the amount of energy released is enormous. The energy obtained from the complete fission of one gram of uranium is approximately equal to the energy obtained from burning 2.5 tons of oil.
Chain reaction of fission of several nuclei
We looked at the fission of one uranium nucleus. During fission, several (usually two or three) neutrons are released. They fly apart at great speed and can easily get into the nuclei of other atoms, causing a fission reaction in them. This is a chain reaction.
That is, the neutrons obtained as a result of nuclear fission excite and force other nuclei to fission, which in turn themselves emit neutrons, which continue to stimulate further fission. And so on until fission of all uranium nuclei in the immediate vicinity occurs.
In this case, a chain reaction can occur avalanche-like, for example, in the event of an atomic bomb explosion. The number of nuclear fissions increases exponentially in a short period of time. However, a chain reaction can also occur with attenuation.
The fact is that not all neutrons meet nuclei on their way, which they induce to fission. As we remember, inside a substance the main volume is occupied by the void between the particles. Therefore, some neutrons fly through all matter without colliding with anything along the way. And if the number of nuclear fissions decreases over time, then the reaction gradually fades.
Nuclear reactions and critical mass of uranium
What determines the type of reaction? From the mass of uranium. The greater the mass, the more particles the flying neutron will meet on its path and the greater the chance of getting into the nucleus. Therefore, a “critical mass” of uranium is distinguished - this is the minimum mass at which a chain reaction is possible.
The number of neutrons produced will be equal to the number of neutrons that fly out. And the reaction will proceed at approximately the same speed until the entire volume of the substance is produced. This is used in practice in nuclear power plants and is called a controlled nuclear reaction.
>> Fission of uranium nuclei
§ 107 FISSION OF URANIUM NUCLEI
Only the nuclei of some heavy elements can be divided into parts. When nuclei fission, two or three neutrons and -rays are emitted. At the same time, a lot of energy is released.
Discovery of uranium fission. The fission of uranium nuclei was discovered in 1938 by German scientists O. Hahn iF. Strassmann. They established that when uranium is bombarded with neutrons, elements of the middle part of the periodic table arise: barium, krypton, etc. However, the correct interpretation of this fact as the fission of a uranium nucleus that captured a neutron was given at the beginning of 1939 by the English physicist O. Frisch together with the Austrian physicist L. Meitner.
Neutron capture disrupts the stability of the nucleus. The nucleus becomes excited and becomes unstable, which leads to its division into fragments. Nuclear fission is possible because the rest mass of a heavy nucleus is greater than the sum of the rest masses of the fragments resulting from fission. Therefore, there is a release of energy equivalent to the decrease in rest mass that accompanies fission.
The possibility of fission of heavy nuclei can also be explained using a graph of the specific binding energy versus mass number A (see Fig. 13.11). The specific binding energy of the nuclei of atoms of elements occupying the last places in the periodic table (A 200) is approximately 1 MeV less than the specific binding energy in the nuclei of elements located in the middle of the periodic system (A 100). Therefore, the process of fission of heavy nuclei into nuclei of elements in the middle part of the periodic table is energetically favorable. After fission, the system enters a state with minimal internal energy. After all, the greater the binding energy of the nucleus, the greater the energy that should be released upon the emergence of the nucleus and, consequently, the less the internal energy of the newly formed system.
During nuclear fission, the binding energy per nucleon increases by 1 MeV and the total energy released must be enormous - on the order of 200 MeV. No other nuclear reaction (not related to fission) releases such large energies.

Direct measurements of the energy released during the fission of a uranium nucleus confirmed the above considerations and gave a value of 200 MeV. Moreover, most of this energy (168 MeV) falls on the kinetic energy of the fragments. In Figure 13.13 you see the tracks of fissile uranium fragments in a cloud chamber.
The energy released during nuclear fission is of electrostatic rather than nuclear origin. The large kinetic energy that the fragments have arises due to their Coulomb repulsion.
Mechanism of nuclear fission. The process of fission of the atomic nucleus can be explained on the basis of the droplet model of the nucleus. According to this model, a bunch of nucleons resembles a droplet of charged liquid (Fig. 13.14, a). Nuclear forces between nucleons are short-range, like the forces acting between liquid molecules. Along with the large forces of electrostatic repulsion between protons, which tend to tear the nucleus into pieces, even greater nuclear forces of attraction act. These forces keep the nucleus from disintegrating.

The uranium-235 nucleus is spherical in shape. Having absorbed an extra neutron, it becomes excited and begins to deform, acquiring an elongated shape (Fig. 13.14, b). The core will stretch until the repulsive forces between the halves of the elongated core begin to prevail over the attractive forces acting in the isthmus (Fig. 13.14, c). After this, it breaks into two parts (Fig. 13.14, d).
Under the influence of Coulomb repulsive forces, these fragments fly apart at a speed equal to 1/30 of the speed of light.
Emission of neutrons during fission. A fundamental fact of nuclear fission is the emission of two to three neutrons during the fission process. It was thanks to this that the practical use of intranuclear energy became possible.
It is possible to understand why free neutrons are emitted based on the following considerations. It is known that the ratio of the number of neutrons to the number of protons in stable nuclei increases with increasing atomic number. Therefore, the relative number of neutrons in fragments arising during fission is greater than is permissible for the nuclei of atoms located in the middle of the periodic table. As a result, several neutrons are released during the fission process. Their energy has different values - from several million electron volts to very small ones, close to zero.
Fission usually occurs into fragments, the masses of which differ by approximately 1.5 times. These fragments are highly radioactive, as they contain an excess amount of neutrons. As a result of a series of successive decays, stable isotopes are eventually obtained.
In conclusion, we note that there is also spontaneous fission of uranium nuclei. It was discovered by Soviet physicists G.N. Flerov and K.A. Petrzhak in 1940. The half-life for spontaneous fission is 10 16 years. This is two million times longer than the half-life of uranium.
The reaction of nuclear fission is accompanied by the release of energy.
Lesson content lesson notes supporting frame lesson presentation acceleration methods interactive technologies Practice tasks and exercises self-test workshops, trainings, cases, quests homework discussion questions rhetorical questions from students Illustrations audio, video clips and multimedia photographs, pictures, graphics, tables, diagrams, humor, anecdotes, jokes, comics, parables, sayings, crosswords, quotes Add-ons abstracts articles tricks for the curious cribs textbooks basic and additional dictionary of terms other Improving textbooks and lessonscorrecting errors in the textbook updating a fragment in a textbook, elements of innovation in the lesson, replacing outdated knowledge with new ones Only for teachers perfect lessons calendar plan for the year; methodological recommendations; discussion programs Integrated LessonsThe fission of uranium nuclei was discovered in 1938 by German scientists O. Hahn and F. Strassmann. They were able to establish that when uranium nuclei are bombarded with neutrons, elements of the middle part of the periodic table are formed: barium, krypton, etc. The correct interpretation of this fact was given by the Austrian physicist L. Meitner and the English physicist O. Frisch. They explained the appearance of these elements by the decay of uranium nuclei that captured a neutron into two approximately equal parts. This phenomenon is called nuclear fission, and the resulting nuclei are called fission fragments.
see also
- Vasiliev A. Uranium fission: from Klaproth to Hahn // Quantum. - 2001. - No. 4. - P. 20-21,30.
Droplet model of the nucleus
This fission reaction can be explained based on the droplet model of the nucleus. In this model, the core is considered as a drop of electrically charged incompressible fluid. In addition to the nuclear forces acting between all nucleons of the nucleus, protons experience additional electrostatic repulsion, as a result of which they are located at the periphery of the nucleus. In an unexcited state, the forces of electrostatic repulsion are compensated, so the nucleus has a spherical shape (Fig. 1, a).

After the \(~^(235)_(92)U\) nucleus captures a neutron, an intermediate nucleus \(~(^(236)_(92)U)^*\) is formed, which is in an excited state. In this case, the neutron energy is evenly distributed among all nucleons, and the intermediate nucleus itself is deformed and begins to vibrate. If the excitation is small, then the nucleus (Fig. 1, b), freeing itself from excess energy by emitting γ -quantum or neutron, returns to a stable state. If the excitation energy is sufficiently high, then the deformation of the core during oscillations can be so great that a constriction is formed in it (Fig. 1, c), similar to the constriction between two parts of a bifurcating drop of liquid. Nuclear forces acting in a narrow waist can no longer withstand the significant Coulomb force of repulsion of parts of the nucleus. The waist breaks, and the core breaks up into two “fragments” (Fig. 1, d), which fly off in opposite directions.
Currently, about 100 different isotopes with mass numbers from about 90 to 145 are known, resulting from the fission of this nucleus. Two typical fission reactions of this nucleus are:
\(~^(235)_(92)U + \ ^1_0n \ ^(\nearrow)_(\searrow) \ \begin(matrix) ^(144)_(56)Ba + \ ^(89)_( 36)Kr + \ 3^1_0n \\ ^(140)_(54)Xe + \ ^(94)_(38)Sr + \ 2^1_0n \end(matrix)\) .
Note that nuclear fission initiated by a neutron produces new neutrons that can cause fission reactions in other nuclei. The fission products of uranium-235 nuclei can also be other isotopes of barium, xenon, strontium, rubidium, etc.
When the nuclei of heavy atoms fission (\(~^(235)_(92)U\)), very large energy is released - about 200 MeV during the fission of each nucleus. About 80% of this energy is released as kinetic energy of fragments; the remaining 20% comes from the energy of radioactive radiation from fragments and the kinetic energy of prompt neutrons.
An estimate of the energy released during nuclear fission can be made using the specific binding energy of nucleons in the nucleus. Specific binding energy of nucleons in nuclei with mass number A≈ 240 of the order of 7.6 MeV/nucleon, while in nuclei with mass numbers A= 90 – 145 specific energy is approximately 8.5 MeV/nucleon. Consequently, the fission of a uranium nucleus releases energy of the order of 0.9 MeV/nucleon, or approximately 210 MeV per uranium atom. The complete fission of all nuclei contained in 1 g of uranium releases the same energy as the combustion of 3 tons of coal or 2.5 tons of oil.
see also
- Varlamov A.A. Droplet model of the nucleus //Quantum. - 1986. - No. 5. - P. 23-24
Chain reaction
Chain reaction- a nuclear reaction in which the particles causing the reaction are formed as products of this reaction.
When a uranium-235 nucleus fissions, which is caused by a collision with a neutron, 2 or 3 neutrons are released. Under favorable conditions, these neutrons can hit other uranium nuclei and cause them to fission. At this stage, from 4 to 9 neutrons will appear, capable of causing new decays of uranium nuclei, etc. Such an avalanche-like process is called a chain reaction. A diagram of the development of a chain reaction of fission of uranium nuclei is shown in Fig. 3.

Uranium occurs in nature in the form of two isotopes \[~^(238)_(92)U\] (99.3%) and \(~^(235)_(92)U\) (0.7%). When bombarded by neutrons, the nuclei of both isotopes can split into two fragments. In this case, the fission reaction \(~^(235)_(92)U\) occurs most intensively with slow (thermal) neutrons, while the nuclei \(~^(238)_(92)U\) react fission only with fast neutrons with energies of the order of 1 MeV. Otherwise, the excitation energy of the resulting nuclei \(~^(239)_(92)U\) turns out to be insufficient for fission, and then nuclear reactions occur instead of fission:
\(~^(238)_(92)U + \ ^1_0n \to \ ^(239)_(92)U \to \ ^(239)_(93)Np + \ ^0_(-1)e\ ) .
Uranium isotope \(~^(238)_(92)U\) β -radioactive, half-life 23 minutes. The neptunium isotope \(~^(239)_(93)Np\) is also radioactive, with a half-life of about 2 days.
\(~^(239)_(93)Np \to \ ^(239)_(94)Pu + \ ^0_(-1)e\) .
The plutonium isotope \(~^(239)_(94)Np\) is relatively stable, with a half-life of 24,000 years. The most important property of plutonium is that it is fissile under the influence of neutrons in the same way as \(~^(235)_(92)U\). Therefore, with the help of \(~^(239)_(94)Np\) a chain reaction can be carried out.
The chain reaction diagram discussed above represents an ideal case. In real conditions, not all neutrons produced during fission participate in the fission of other nuclei. Some of them are captured by the non-fissile nuclei of foreign atoms, others fly out of the uranium (neutron leakage).
Therefore, a chain reaction of fission of heavy nuclei does not always occur and not for any mass of uranium.
Neutron multiplication factor
The development of a chain reaction is characterized by the so-called neutron multiplication factor TO, which is measured by the ratio of the number N i neutrons causing fission of the nuclei of a substance at one of the stages of the reaction, to the number N i-1 neutrons that caused fission at the previous stage of the reaction:
\(~K = \dfrac(N_i)(N_(i - 1))\) .
The multiplication coefficient depends on a number of factors, in particular on the nature and quantity of the fissile substance, and on the geometric shape of the volume it occupies. The same amount of a given substance has different meanings TO. TO maximum if the substance has a spherical shape, since in this case the loss of prompt neutrons through the surface will be minimal.
The mass of a fissile substance in which a chain reaction occurs with a multiplication factor TO= 1 is called critical mass. In small pieces of uranium, most neutrons fly out without hitting any nucleus.
The value of the critical mass is determined by the geometry of the physical system, its structure and external environment. Thus, for a ball of pure uranium \(~^(235)_(92)U\) the critical mass is 47 kg (a ball with a diameter of 17 cm). The critical mass of uranium can be reduced many times by using so-called neutron moderators. The fact is that neutrons produced during the decay of uranium nuclei have too high speeds, and the probability of capturing slow neutrons by uranium-235 nuclei is hundreds of times greater than fast ones. The best neutron moderator is heavy water D 2 O. When interacting with neutrons, ordinary water itself turns into heavy water.
Graphite, whose nuclei do not absorb neutrons, is also a good moderator. During elastic interaction with deuterium or carbon nuclei, neutrons are slowed down to thermal speeds.
The use of neutron moderators and a special beryllium shell, which reflects neutrons, makes it possible to reduce the critical mass to 250 g.
At the multiplication rate TO= 1 the number of fissioning nuclei is maintained at a constant level. This mode is provided in nuclear reactors.
If the mass of nuclear fuel is less than the critical mass, then the multiplication factor TO < 1; каждое новое поколение вызывает все меньшее и меньшее число делений, и реакция без внешнего источника нейтронов быстро затухает.
If the mass of nuclear fuel is greater than the critical mass, then the multiplication factor TO> 1 and each new generation of neutrons causes an increasing number of fissions. The chain reaction grows like an avalanche and has the character of an explosion, accompanied by a huge release of energy and an increase in the ambient temperature to several million degrees. This kind of chain reaction occurs when an atomic bomb explodes.
Nuclear bomb
In its normal state, a nuclear bomb does not explode because the nuclear charge in it is divided into several small parts by partitions that absorb the decay products of uranium - neutrons. The nuclear chain reaction that causes a nuclear explosion cannot be sustained under such conditions. However, if fragments of a nuclear charge are combined together, their total mass will become sufficient for a chain reaction of uranium fission to begin to develop. The result is a nuclear explosion. Moreover, the explosion power developed by a relatively small nuclear bomb is equivalent to the power released during the explosion of millions and billions of tons of TNT.

Rice. 5. Atomic bomb