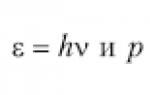De Broglie's hypothesis formulation. De Broglie's hypothesis and formula. Experimental confirmation of the hypothesis. Dual particle-wave nature
A). The French scientist Louis de Broglie (1892–1987) in 1924, in his doctoral dissertation “Research on Quantum Theory,” put forward a bold hypothesis about the universality of wave-particle duality, arguing that since light behaves in some cases as a wave, and in others - as a particle, then material particles (electrons, etc.), due to the generality of the laws of nature, must have wave properties. “In optics,” he wrote, “for a century, the corpuscular method of examination was too neglected in comparison with the wave one; Wasn't the opposite mistake made in the theory of matter? Have we thought too much about the “particle” picture and neglected the wave picture too much?” At the time, de Broglie's hypothesis seemed crazy. Only in 1927, three years later, did science experience a huge shock: physicists K. Davisson and L. Germer experimentally confirmed de Broglie’s hypothesis by obtaining a diffraction pattern of electrons.
According to A. Einstein’s quantum theory of light, the wave characteristics of photons of light (oscillation frequency v f, relativistic mass m f and momentum p f) relations:
According to de Broglie's idea, any microparticle, including one with a rest mass w 0 C 0, must have not only corpuscular, but also wave properties. Corresponding Frequency v and wavelength l are determined by relations similar to Einstein’s:
![]()
Hence the de Broglie wavelength is
Thus, Einstein’s relations, obtained by him when constructing the theory of photons as a result of the hypothesis put forward by de Broglie, acquired a universal character and became equally applicable both for the analysis of the corpuscular properties of light and for the study of the wave properties of all microparticles.
B). Light has both wave and corpuscular properties. Wave properties appear during the propagation of light (interference, diffraction). Corpuscular properties appear during the interaction of light with matter (photoelectric effect, emission and absorption of light by atoms).
Properties of a photon as a particle (energy E and momentum p) are related to its wave properties (frequency ν and wavelength λ) by the relations
Where h= 6.63·10 –34 J·s – Planck’s constant.
The French physicist de Broglie suggested in 1924 that the combination of wave and corpuscular properties is inherent not only in light, but also in any material body. According to de Broglie, every body with a mass m, moving with speed v, corresponds to a wave process with wavelength
(nonrelativistic approximation υ<< c).
Wave properties are most clearly manifested in elementary particles. This happens because, due to the small mass of the particles, the wavelength is comparable to the distance between atoms in crystal lattices. In this case, when a beam of particles interacts with a crystal lattice, diffraction occurs.
To illustrate the wave properties of particles, a thought experiment is often used - the passage of a beam of electrons (or other particles) through a slit of width Δ x. From the point of view of wave theory, during diffraction by a slit, the beam will broaden with an angular divergence θ ≥ λ / Δ x. From a corpuscular point of view, the broadening of the beam after passing through the slit is explained by the appearance of a certain transverse momentum in the particles. The spread of values of this transverse momentum (“uncertainty”) is
Ratio
is called the uncertainty relation. This relationship in corpuscular language expresses the presence of wave properties in particles.
An experiment involving the passage of a beam of electrons through two closely spaced slits can serve as an even more striking illustration of the wave properties of particles. This experiment is an analogue of Young's optical interference experiment.
The computer model recreates on the display screen thought experiments on electron diffraction by one and two slits.
When approaching a screen with slits, particles interact with it like de Broglie waves. The behavior of particles in the space between a screen with slits and a photographic plate is described in quantum physics using Ψ functions. The square of the modulus of the psi function determines the probability of detecting a particle in a particular location. Thus, the arrival of particles at various points on a photographic plate is a probabilistic process. A computer model allows you to demonstrate this process.
In the case of a single slit, the model illustrates the uncertainty relationship that is a consequence of the dual nature of the particles. You can change the width of the slit within certain limits and observe the diffraction blurring of the electron beam on the photographic plate.
It is assumed that electrons have an energy of the order of 100 eV.
Note that in the case of two slits, the distribution observed on the photographic plate is not a simple superposition of two independent distributions from each of the slits separately. The appearance of interference fringes on the photographic plate clearly indicates that each particle that reached the photographic plate simultaneously passed through both slits of the screen.
64.Heisenberg uncertainty relation. In 1927, W. Heisenberg discovered the so-called , according to which the uncertainties of position and momentum are related to each other by the relation: , where, h Planck's constant. The uniqueness of the description of the microworld is that the product of the uncertainty (accuracy of determination) of the position Δx and the uncertainty (accuracy of determination) of the momentum Δp x must always be equal to or greater than a constant equal to – . It follows from this that a decrease in one of these quantities should lead to an increase in the other. It is well known that any measurement is associated with certain errors, and by improving measuring instruments, it is possible to reduce errors, i.e., increase measurement accuracy. But Heisenberg showed that there are conjugate (additional) characteristics of a microparticle, the exact simultaneous measurement of which is fundamentally impossible. Those. uncertainty is a property of the state itself; it is not related to the accuracy of the device.
For other conjugate quantities - energy E and time t uncertainty relations, has the form: . This means that at the characteristic evolution time of the system Δ t, the error in determining its energy cannot be less than . From this relationship follows the possibility of the emergence from nothing of the so-called virtual particles for a period of time less than those with energy Δ E. In this case, the law of conservation of energy will not be violated. Therefore, according to modern ideas vacuumit is not a void in which fields and particles are absent, but a physical entity in which virtual particles constantly appear and disappear.
One of the basic principles of quantum mechanics is uncertainty principle, discovered by Heisenberg. Obtaining information about some quantities that describe a microobject inevitably leads to a decrease in information about other quantities, additional to the first. Instruments that record quantities related by uncertainty relations are of different types; they are complementary to each other. By measurement in quantum mechanics we mean any process of interaction between classical and quantum objects that occurs in addition to and independently of any observer. If in classical physics a measurement did not disturb the object itself, then in quantum mechanics each measurement destroys the object, destroying its wave function. For a new measurement, the object must be prepared again. In this regard, N. Bohr put forward principle of complementarity, the essence of which is that for a complete description of the objects of the microworld it is necessary to use two opposite, but complementary, representations.
Photon diffraction as an illustration of the uncertainty relationship
From the point of view of quantum theory, light can be considered as a stream of light quanta - photons. When a monochromatic plane wave of light is diffraction on a narrow slit, each photon passing through the slit hits a certain point on the screen (Fig. 1.). It is impossible to predict exactly where the photon will hit. However, in the aggregate, when photons hit different points on the screen, they give a diffraction pattern. When a photon passes through a slit, we can say that its x coordinate has been determined with an error Δx, which is equal to the size of the slit. If the front of a plane monochromatic wave is parallel to the plane of the screen with a slit, then each photon has a momentum directed along the z axis perpendicular to the screen. Knowing the wavelength, this pulse can be accurately determined: p = h/λ.
However, after passing through the slit, the direction of the pulse changes, as a result of which a diffraction pattern is observed. The pulse modulus remains constant, since the wavelength does not change during light diffraction. Deviation from the original direction occurs due to the appearance of the component Δp x along the x axis (Fig. 1.). The value of this component for each specific photon cannot be determined, but its maximum absolute value determines the width of the 2S diffraction pattern. The maximum value Δp x is a measure of the uncertainty of the photon momentum that arises when determining its coordinates with an error Δx. As can be seen from the figure, the maximum value of Δp x is equal to: Δp x = psinθ, . If L>> s , then we can write: sinθ =s/ L and Δp x = p(s/ L).
The insufficiency of Bohr's theory pointed to the need to revise the foundations of quantum theory and ideas about the nature of microparticles (electrons, protons, etc.). The question arose about how comprehensive the representation of the electron in the form of a small mechanical particle, characterized by certain coordinates and a certain speed, is.
As a result of deepening ideas about the nature of light, it became clear that a kind of dualism is revealed in optical phenomena. Along with such properties of light that most directly indicate its wave nature (interference, diffraction), there are other properties that just as directly reveal its corpuscular nature (photoelectric effect, Compton phenomenon).
In 1924, Louis de Broglie put forward a bold hypothesis that dualism is not a feature of optical phenomena alone, but has a universal significance. “In optics,” he wrote, “for a century, the corpuscular method of examination was too neglected in comparison with the wave one; Wasn’t the opposite mistake made in the theory of matter?” Assuming that particles of matter, along with corpuscular properties, also have wave properties, de Broglie transferred to the case of particles of matter the same rules of transition from one picture to another that are valid in the case of light. A photon has energy
and impulse
According to de Broglie's idea, the movement of an electron or any other particle is associated with a wave process, the wavelength of which is equal to
![]()
and frequency
De Broglie's hypothesis was soon confirmed experimentally. Davisson and Germer studied in 1927 the reflection of electrons from a nickel single crystal belonging to the cubic system.
A narrow beam of monoenergetic electrons was directed onto the surface of a single crystal, ground perpendicular to the large diagonal of the crystal cell (crystal planes parallel to this surface are designated in crystallography by the indices (111); see § 45). The reflected electrons were collected by a cylindrical electrode attached to a galvanometer (Fig. 18.1). The intensity of the reflected beam was estimated from the current flowing through the galvanometer. The electron speed and angle were varied. In Fig. Figure 18.2 shows the dependence of the current measured by a galvanometer on the angle at various electron energies.


The vertical axis in the graphs determines the direction of the incident beam. The current strength in a given direction is represented by the length of a segment drawn from the origin to the intersection with the curve. It can be seen from the figure that the scattering turned out to be especially intense at a certain angle. This angle corresponded to reflection from atomic planes, the distance between which d was known from x-ray studies. At this, the current strength turned out to be especially significant at an accelerating voltage of 54 V. The wavelength corresponding to this voltage, calculated using formula (18.1), is equal to 1.67 A.
Bragg wavelength satisfying the condition
![]()
was equal to 1.65 A. The coincidence is so striking that the experiments of Davisson and Germer should be recognized as a brilliant confirmation of de Broglie’s idea.
G. P. Thomson (1927) and, independently of him, P. S. Tartakovsky obtained a diffraction pattern when an electron beam passed through metal foil. The experiment was carried out as follows (Fig. 18.3). A beam of electrons, accelerated by a potential difference of the order of several tens of kilovolts, passed through a thin metal foil and fell on a photographic plate. When an electron hits a photographic plate, it has the same effect on it as a photon. The electron diffraction pattern of gold obtained in this way (Fig. 18.4, a) is compared with the x-ray diffraction pattern of aluminum obtained under similar conditions (Fig. 18.4, b).


The similarity of both pictures is striking; Stern and his collaborators showed that diffraction phenomena are also found in atomic and molecular beams. In all of the above cases, the diffraction pattern. corresponds to the wavelength determined by relation (18.1).
In the experiments of Davisson and Germer, as well as in the experiments of Thomson, the intensity of the electron beams was so great that a large number of electrons passed through the crystal simultaneously. Therefore, it could be assumed that the observed diffraction pattern is due to the simultaneous participation of a large number of electrons in the process, and an individual electron passing through the crystal does not detect diffraction. To clarify this issue, Soviet physicists L.M. Biberman, N.G. Sushkin and V.A. Fabrikant carried out an experiment in 1949 in which the intensity of the electron beam was so weak that the electrons passed through the device one by one. The time interval between two successive passages of electrons through the crystal was approximately 30,000 times greater than the time it took an electron to travel through the entire device. With sufficient exposure, a diffraction pattern was obtained that was no different from that observed at normal beam intensity. Thus, it was proven that wave properties are inherent in an individual electron.
The insufficiency of Bohr's theory made it necessary to critically revise the foundations of quantum theory and ideas about the nature of elementary particles (electrons, protons, etc.). The question arose about how comprehensive the representation of the electron in the form of a small mechanical particle, characterized by certain coordinates and a certain speed, is.
As a result of deepening our knowledge about the nature of light, it became clear that a kind of dualism is revealed in optical phenomena (see § 57). Along with such properties of light that most directly indicate its wave nature (interference, diffraction), there are other properties that just as directly reveal its corpuscular nature (photoelectric effect, Compton phenomenon).
In 1924, Louis de Broglie put forward a bold hypothesis that duality is not a feature of optical phenomena alone, but has universal significance. “In optics,” he wrote, “for a century, the corpuscular method of examination was too neglected in comparison with the wave one; hasn’t the opposite mistake been made in the theory of matter?”
Assuming that particles of matter, along with corpuscular properties, also have wave properties, de Broglie transferred the same rules of translation to the case of particles of matter.
transition from one picture to another, which is true in the case of light. Photon, as is known [see. formulas (57.1) and (57.4)], has energy
and impulse
According to de Broglie's idea, the movement of an electron or any other particle is associated with a wave process, the wavelength of which is equal to
and frequency
De Broglie's hypothesis was soon brilliantly confirmed experimentally. Davisson and Germer discovered that a beam of electrons scattered from a crystal plate produces a diffraction pattern. Thomson and, independently, Tartakovsky obtained a diffraction pattern when an electron beam passed through metal foil. The experiment was carried out as follows (Fig. 190). A beam of electrons, accelerated by a potential difference of the order of several tens of kilovolts, passed through a thin metal foil and fell on a photographic plate. When an electron hits a photographic plate, it has the same effect on it as a photon. The electron diffraction pattern of gold obtained in this way (Fig. 191, A) compared with an x-ray diffraction pattern of aluminum obtained under similar conditions (Fig. 191.6). The similarities between both paintings are striking.
Stern and his co-workers showed that diffraction phenomena are also found in atomic and molecular beams. In all of the above cases


the diffraction pattern corresponds to the wavelength determined by relation (64.1).
From the described experiments it undoubtedly follows that a beam of microparticles of a certain speed and

■control gives a diffraction pattern similar to the pattern obtained from a plane wave.
Electron diffraction - scattering process electrons on a collection of particles of a substance in which the electron exhibits wave properties. This phenomenon is called wave-particle duality, in the sense that a particle of matter (in this case, interacting electrons) can be described as a wave.
NEUTRON DIFFRACTION- the phenomenon of neutron scattering, in which the wave properties of the neutron play a decisive role (see. Wave-particle duality).Wavelength and momentum r related by de Broglie's relation =hp. Math. the description of D. n., as well as in the case of other wave fields, follows from Huygens-Fresnel principle and, in this sense, similar to the description light diffraction, x-ray rays, electrons and other microparticles (see. Wave diffraction).According to this description, the intensity of the scattered radiation at a certain point in space depends both on and on the properties of the scattering object. Accordingly, D. n. used both for studying or forming neutron beams (neutron monochromators, analyzers), and for studying the structure of the scattering substance.

Rice. 1. Angular distribution of neutrons with an energy of 14 MeV scattered on the Sn nucleus; - scattering cross section; - scattering angle.
Estimation of the zero-point energy of the oscillator. We will act in exactly the same way as in the previous example. The energy of a classical one-dimensional harmonic oscillator is described by the expression
E = px2 / 2m + mω2x2 / 2.
Considering px and x as uncertainties in the momentum and coordinates of an oscillating microobject and using the equality pxх = h as the uncertainty relation, we obtain
E(px) = px2 / 2m + mω2h2 / 2px2.
Equating the derivative to zero, we find the quantity
p0 = mωh, at which the function E(px) takes on a minimum value. It is easy to verify that this value is equal to
E = E(p0) = hω.
This result is quite interesting. It shows that in quantum mechanics the oscillator energy cannot vanish; its minimum value turns out to be of the order of hω. This is the so-called zero-point energy.
Taking into account the existence of zero-point vibrations, we can come, in particular, to the following interesting conclusion: the energy of the vibrational motion of the atoms of a crystal does not vanish even at absolute zero temperature.
Zero oscillations illustrate a fundamental general circumstance: it is impossible to realize a microobject at the “bottom of a potential well,” or, in other words, “a microobject cannot fall to the bottom of a potential well.” This conclusion does not depend on the type of potential well, since it is a direct consequence of the momentum uncertainty relations; in this case, the uncertainty of the coordinate should become arbitrarily large, which contradicts the very fact that the microobject is in the potential well.
Electron tunneling through a potential barrier is a fundamentally quantum mechanical effect that has no analogue in classical mechanics. The tunnel effect is an experimental confirmation of one of the fundamental starting points of quantum mechanics - the wave-particle duality of the properties of elementary particles.
The tunnel effect is the ability of an elementary particle, such as an electron, to pass (tunnel) through a potential barrier when the barrier is higher than the total energy of the particle. The possibility of the existence of a tunnel effect in the microcosm was understood by physicists during the creation of quantum mechanics, in the 20-30s of our century. Subsequently, due to the tunnel effect, some very important phenomena discovered experimentally in various fields of physics were explained.
Question 12
Atom (from Old Greekἄτομος - indivisible) - a particle of a substance of microscopic size and mass, the smallest part chemical element, which is the bearer of its properties.
An atom is made up of atomic nucleus And electrons. If the number of protons in the nucleus coincides with the number of electrons, then the atom as a whole turns out to be electrically neutral. Otherwise it has some positive or negative charge and is called ion. In some cases, atoms are understood only as electrically neutral systems, in which the charge of the nucleus is equal to the total charge of the electrons, thereby contrasting them with electrically charged ions.
The nucleus, which carries almost the entire (more than 99.9%) mass of the atom, consists of positively charged protons and uncharged neutrons, connected to each other using strong interaction. Atoms are classified according to the number of protons and neutrons in the nucleus: the number of protons Z corresponds to the atomic number in in the periodic table and determines its belonging to a certain chemical element, and the number of neutrons N - a certain isotope this element. The Z number also determines the total positive electric charge (Z e) of the atomic nucleus and the number of electrons in a neutral atom, which determines its size.
HYDROGEN-LIKE ATOMS- atoms (ions), consisting, like a hydrogen atom, of a nucleus and one electron. These include ions of elements with at. number 2, having lost all electrons except one: He +, Li +2, B+ 3,. . . Together with hydrogen they form the simplest isoelectronic series.Energy levels (and spectra) of V. a. are similar to hydrogen ones, differing from them in the scale of energies (and frequencies) of transitions by a factor of Z 2 (see. Atom).
Systems similar to V. a. form an atomic nucleus and a meson ( mesoatom), as well as electron and positron ( positronium; ) for these systems energy levels and spectra similar to hydrogen are also obtained.
Energy level - eigenvalues energies of quantum systems, that is, systems consisting of microparticles ( electrons, protons and others elementary particles) and subject to laws quantum mechanics. Each level is characterized by a certain state of the system, or a subset of those in the case degeneration. The concept applies to atoms(electronic levels), molecules(various levels corresponding to oscillations and rotations), atomic nuclei(intranuclear energy levels), etc.
Ionization and excitation.
To free an electron from its bond with the atomic nucleus, resulting in the formation of a positive ion, it is necessary to expend a certain amount of energy. The energy expended to remove an electron is called ionization work. The work of ionization, expressed in electron volts, is called ionization potential(electronvolt is a unit of energy acquired by an electron accelerated by an electric field with a potential difference of 1 V). If you impart a certain amount of additional energy to the bound electron of a gas molecule or atom, the electron will move to a new orbit with a higher energy level, and the molecule or atom will be in an excited state. The amount of energy, expressed in electron volts, that must be expended to excite an atom or molecule of a gas is called excitation potential. The excited state of an atom or molecule of a gas is unstable, and the electron can again return to a stationary orbit, and the atom or molecule will go into a normal unexcited state. The excitation energy is transmitted to the surrounding space in the form of light electromagnetic radiation.
The magnitude of the ionization and excitation potential depends on the nature of the atom. Lowest ionization potential
(3.9 eV) have cesium vapor, and the highest (24.5 eV) is observed for helium gas. Alkaline earth metals (cesium, potassium, sodium, barium, calcium) have a weak connection between electrons and the nucleus, so they have the lowest ionization potentials, therefore, less energy will be required to excite and work function of the electron than iron, manganese, copper and nickel . Elements that have lower ionization and excitation potentials than the metal being welded are introduced into the composition of electrode coatings in order to increase the stabilization of the arc discharge in gases. The amount of energy required to release an electron from a metal or liquid is called electron work function and is expressed in electronvolts.
Spatial distribution of an electron in a hydrogen atom. @
Graphically, the probability of finding an electron can be depicted as a cloud, where darker areas correspond to a higher probability of finding. The "size" and "shape" of the electron cloud in a given atomic state can be calculated. For the ground state of the hydrogen atom, solving the Schrödinger equation gives  , (2.6)
, (2.6)
Where φ
(r) is a wave function that depends only on the distance r to the center of the atom, r 1 is a constant that coincides with the radius of the first Bohr orbit. Consequently, the electron cloud in the ground state of hydrogen is spherically symmetric, as shown in Figure 11. The electron cloud only approximately characterizes the size of the atom and the motion of the electron, since according to (2.15) the probability of detecting an electron is not zero for any point in space. Figure 12 shows the electron clouds of the hydrogen atom in the states: n=2, l=1 and m=1, 0, -1 in the presence of a magnetic field.

Rice. 11. Electron cloud of a hydrogen atom in the ground state n = 1, l = 0.

Rice. 12. Electron clouds of the hydrogen atom and precession of angular momentum in states n = 2, l = 1 for m = 1, 0, -1
If in these states we determine the most probable distances of the electron from the nucleus, then they will be equal to the radii of the corresponding Bohr orbits. Thus, although quantum mechanics does not use the idea of electron motion along certain trajectories, nevertheless, the radii of Bohr orbits in this theory can be given a certain physical meaning.
LEVEL WIDTH- uncertainty of energy quantum-mechanical. system (atom, molecule, etc.) that has discrete energy levels in a state that is not strictly stationary. Sh.u. D, which characterizes the blurring of the energy level, its broadening, depends on cf. duration of stay of the system in a given state - lifetime at level t k and, according to uncertainty relationship for energy and time, For a strictly stationary state of the system t k= and D =0. Lifetime t k, and therefore Sh.u. due to the possibility quantum transitions systems into states with other energies. For a free system (for example, for an isolated atom) spontaneous emissions. transitions from a level to lower levels determine the radiation, or natural, level:
![]() , where is the total probability of spontaneous emission from the level, Aki- Einstein coefficients for spontaneous emission. Level broadening can also be caused by spontaneous non-emissions. transitions, for example for radioact. atomic nucleus - alpha decay
.The width of an atomic level is very small compared to the energy of the level. In other cases (for example, for excited nuclei, the probability of quantum transitions is due to the emission of neutrons and is very high) Sh.u. may become comparable to the distance between levels. Any interactions that increase the probability of the system transitioning to other states lead to additional conditions. broadening of levels. An example is the broadening of the levels of an atom (ion) in plasma as a result of its collision with ions and electrons (see. Plasma radiation)
. In general, the total Sh.u. proportional the sum of the probabilities of all possible transitions from this level - spontaneous and caused by decomposition. interactions.
, where is the total probability of spontaneous emission from the level, Aki- Einstein coefficients for spontaneous emission. Level broadening can also be caused by spontaneous non-emissions. transitions, for example for radioact. atomic nucleus - alpha decay
.The width of an atomic level is very small compared to the energy of the level. In other cases (for example, for excited nuclei, the probability of quantum transitions is due to the emission of neutrons and is very high) Sh.u. may become comparable to the distance between levels. Any interactions that increase the probability of the system transitioning to other states lead to additional conditions. broadening of levels. An example is the broadening of the levels of an atom (ion) in plasma as a result of its collision with ions and electrons (see. Plasma radiation)
. In general, the total Sh.u. proportional the sum of the probabilities of all possible transitions from this level - spontaneous and caused by decomposition. interactions.
Features of the structure of electronic levels in complex atoms. Relationship between the distribution of electrons in orbitals and the periodic table of Mendeleev. @
Conventionally, all possible quantum states are distributed (grouped) into layers (shells), sublayers (subshells) and orbitals. As it turned out, the properties of atoms are determined by the distribution of electrons over these states.
A quantum layer (quantum shell) is a set of states that correspond to the same value of the quantum number n, but different values of l, m, s. The largest number of electrons N that can be in the shell, according to (2.8), is equal to twice the square of the layer number: N=2n 2 . Since the energy of states in a multielectron atom depends on two quantum numbers n and l, electrons in the quantum layer can occupy l energy levels. Quantum layers are designated by numbers corresponding to the layer numbers, in addition, they have names: layer n = 1 is called the K layer (or K shell), layer n = 2 is called the L layer (or L shell), layer n = 3 is called the M layer, n = 4 – N, n = 5 – O layer, n = 6 – P and so on.
Each quantum layer with number n conditionally consists of n quantum sublayers (subshells), corresponding to states with the same n, l, but different m, s. A sublayer can contain up to 2(2l+1 ) electrons, sublayers are designated by letters: l = 0 – s, l= 1 – p, l= 2 – d, l= 3 – f, l= 4 – g, etc. The energy of electrons in one sublayer is approximately the same.
In turn, each sublayer consists of 2l+1 orbitals, corresponding to states with the same n, l, m, but different s. 1/2.±Each orbital can contain no more than two electrons with different spin numbers s =
It follows that the s-sublayer can contain a maximum of 2 electrons, the p-sublayer - 6, d - 10, f - 14, g - 18 electrons. Accordingly, the K layer can contain a maximum of 2 electrons, the L layer – 8, the M layer – 18, the N layer – 32, etc.
1s® Structures and the maximum possible fillings of layers are depicted in the form of formulas: K-layer 2 2s®, L layer 2 2p 6 3s®, M-layer 2 3p 6 3d 10 4s®, N-layer 2 4p 6 4d 10 4f 14. Using the introduced concepts, you can conventionally use a formula and graphically depict the distribution of electrons, for example, the oxygen atom O 8, as follows: symbolically - 1s 2 2s 2 2p 4, graphically - (Fig. 14).

Fig. 14. Conventional graphical representation of oxygen orbitals.
When populating orbitals, electrons are first located singly in each orbital, and then they begin to be filled with second electrons. This feature is called Hund’s rule; it is due to the fact that the energy of the sublayer with such filling is somewhat lower. Figure 14 shows the application of this rule to oxygen.
The Pauli principle is a fundamental law of nature, according to which in a quantum system two (or more) identical particles with half-integer spin cannot simultaneously be in the same state. Formulated by W. Pauli (1925).
The state of each electron in an atom is characterized by four quantum numbers:
1. Principal quantum number n (n = 1, 2 ...).
2. Orbital (azimuthal) quantum number l (l = 0, 1, 2, ... n-1).
3. Magnetic quantum number m (m = 0, +/-1, +/-2, +/-... +/-l).
4. Spin quantum number ms (ms = +/-1/2).
For one fixed value of the principal quantum number n, there are 2n2 different quantum states of the electron.
One of the laws of quantum mechanics, called the Pauli principle, states:
In the same atom there cannot be two electrons that have the same set of quantum numbers (that is, there cannot be two electrons in the same state).
The Pauli principle provides an explanation for the periodic repetition of the properties of the atom, i.e. Mendeleev's periodic system of elements.
Bohr's first postulate (postulate of stationary states) states: an atomic system can only be in special stationary or quantum states, each of which corresponds to a certain energy En. In stationary states, the atom does not radiate.
This postulate is in clear contradiction with classical mechanics, according to which the energy of a moving electron can be any. It also contradicts electrodynamics, since it allows for the possibility of accelerated movement of electrons without emitting electromagnetic waves. According to Bohr's first postulate, an atom is characterized by a system energy levels , each of which corresponds to a specific stationary state (Fig. 6.2.2). The mechanical energy of an electron moving along a closed path around a positively charged nucleus is negative. Therefore, all stationary states correspond to energy values E n < 0. При E n≥ 0 the electron moves away from the nucleus, i.e. ionization occurs. Magnitude | E 1 | called ionization energy . State of energy E 1 is called underlying condition atom.
Bohr's second postulate (frequency rule) is formulated as follows: when an atom transitions from one stationary state with energy E n to another stationary state with energy E m, a quantum is emitted or absorbed, the energy of which is equal to the difference in the energies of the stationary states:
Bohr's second postulate also contradicts Maxwell's electrodynamics, since the frequency of radiation is determined only by the change in the energy of the atom and does not depend in any way on the nature of the electron’s movement.
Bohr's theory, when describing the behavior of atomic systems, did not completely reject the laws of classical physics. It preserved the ideas about the orbital motion of electrons in the Coulomb field of the nucleus. The classical nuclear model of the Rutherford atom in Bohr's theory was supplemented by the idea of quantization of electron orbits. Therefore Bohr's theory is sometimes called semi-classical .
LINE SPECTRA - optical emission and absorption spectra, consisting of individual spectral lines. L.S. are atomic spectra, spectra of stellar atmospheres (see Fraunhofer lines), spectra of organic. molecules at low pax temps in special. conditions (see...
ATOMIC SPECTRA - optical spectra of free or weakly bound atoms (monatomic gases, vapors). Caused by quantum transitions of the atom. Atomic spectra are line spectra, consisting of individual spectral lines, which are characterized by a certain length waves and for simple atoms they are grouped into spectral series. They contain information about the structure of atoms and are also used in spectral analysis.
Question 13.
ATOMIC NUCLEUS - the central massive part of an atom, consisting of protons and neutrons (nucleons). In Ya. a. almost the entire mass of the atom is concentrated (more than 99.95%). The dimensions of the nuclei are about 10 -13 -10 -12 cm. The nuclei have a positive electric charge, multiple of abs. electron charge value e: Q = Ze. The integer Z matches the ordinal number of the element in periodic table of elements . Ya. a. was discovered by E. Rutherford in 1911 in experiments on the scattering of alpha particles as they passed through matter.
STRUCTURE
The nucleus is the central part of an atom. The positive electric charge and the bulk of the mass of the atom are concentrated in it; Compared to the radius of electron orbits, the dimensions of the nucleus are extremely small: 10-15 - 10-14 m. The nuclei of all atoms consist of protons and neutrons, which have almost the same mass, but only the proton carries an electric charge. The total number of protons is called the atomic number Z of an atom, which is the same as the number of electrons in a neutral atom. Nuclear particles (protons and neutrons), called nucleons, are held together by very strong forces; By their nature, these forces can be neither electrical nor gravitational, and in magnitude they are many orders of magnitude greater than the forces that bind electrons to the nucleus. The first idea of the true size of the nucleus was provided by Rutherford's experiments on the scattering of alpha particles in thin metal foils. The particles penetrated deeply through the electron shells and were deflected as they approached the charged nucleus. These experiments clearly indicated the small size of the central nucleus and indicated a method for determining the nuclear charge. Rutherford found that alpha particles approached the center of the positive charge at a distance of about 10-14 m, and this allowed him to conclude that this was the maximum possible radius of the nucleus. Based on these assumptions, Bohr built his quantum theory of the atom, which successfully explained discrete spectral lines, the photoelectric effect, X-rays, and the periodic table of elements. However, in Bohr's theory the nucleus was considered as a positive point charge. The nuclei of most atoms turned out to be not only very small, but they were not affected in any way by such means of exciting optical phenomena as an arc spark discharge, flame, etc. An indication of the presence of a certain internal structure of the nucleus was the discovery of radioactivity in 1896 by A. Becquerel. It turned out that uranium, and then radium, polonium, radon, etc. emit not only short-wave electromagnetic radiation, x-rays and electrons (beta rays), but also heavier particles (alpha rays), and these could only come from the massive part of the atom. Rutherford used radium alpha particles in his scattering experiments, which served as the basis for the formation of ideas about the nuclear atom. (At that time it was known that alpha particles are helium atoms stripped of their electrons; but the question of why some heavy atoms spontaneously emit them was not yet answered, nor was there an accurate idea of the size of the nucleus.)
Kernel Models
Beginning The period of development of nuclear physics is associated with the formation and development of droplet and shell models of the nucleus. These Ya. M. arose almost simultaneously in the 30s. 20th century They are based on various representations and are intended to describe the opposite properties of nuclei. In the droplet model, the core is considered as a continuous medium consisting of neutron and proton liquids and described by classical equations. hydrodynamics (hence the other name - hydrodynamics). Density nuclear liquid is almost constant inside the volume of the drop and drops sharply in the surface layer, the thickness of which is significantly less than the radius of the drop. Basic parameters: equilibrium density of boundless nuclear liquid r 0 (0.16 particles/fm 3), binding energy per 1 nucleon m 0 (16 MeV) and coefficient. surface tension s (1 MeV/fm 2); sometimes s 1 and s 2 are introduced for neutrons and protons separately. To take into account the dependence of the nuclear binding energy on the value of the neutron excess ( N-Z; N And Z- respectively, the number of neutrons and protons in the nucleus), an isovector coefficient is introduced. compressibility of nuclear matter b (30 MeV); to take into account the finite compressibility of nuclear matter - isoscal coefficient. compressibility (compression modulus) K(200 MeV).
Droplet model of the nucleus describes the basic macroscopic properties of nuclei: saturation property, i.e. proportionality of the binding energy of heavy nuclei to the mass number A = N+Z; dependence of the core radius R on A: R = r 0 A 1/3, where r 0 is an almost constant coefficient. (1.06 fm) with the exception of the lightest nuclei. It leads to the Weizsacker formula, which on average describes the binding energies of nuclei well. The droplet model describes nuclear fission well. In combination with the so-called. shell correction (see below), it still serves as the basis. tool for studying this process.
The shell model of the nucleus is based on the idea of the nucleus as a system of nucleons moving independently in a medium. field of the nucleus created by the force action of the remaining nucleons. This nuclear model arose by analogy with the atomic model of shells and was originally intended to explain the experimentally discovered deviations from the Weizsäcker formula and the existence magical nuclei, for which N and Z correspond to the most. pronounced maxima of binding energy. Unlike the droplet model, which almost immediately appeared in its finished form, the shell model underwent a long period of development. search period opt-tim. potential forms cf. field U(r), providing the correct values of the magic. numbers. The decisive step was taken in the end. 40s M. Goeppert-Mayer and H. Jensen, who discovered the important role of the spin-orbit term (U SL)avg. fields. For the center parts of the core in modern times. theories usually use the Saxon-Woods potential.
NUCLEAR REACTIONS
NUCLEAR REACTIONS, transformations of atomic nuclei when interacting with elementary particles, g-quanta or with each other. Nuclear reactions are used in experimental nuclear physics (studying the properties of elementary particles, obtaining transuranium elements, etc.), extracting and using nuclear energy, etc. Nuclear reactions are the main process of producing energy from luminous stars.
POROGREACTIONS 
Mechanisms of nuclear reactions.
According to the mechanism of interaction, nuclear reactions are divided into two main types:
Reactions with the formation of a compound nucleus are a two-stage process that occurs at low temperatures.
high kinetic energy of colliding particles (up to approximately 10 MeV).
Direct nuclear reactions that take place in the nuclear time required for the particle to
crossed the core. This mechanism mainly manifests itself at very high energies of bombarding particles.
From the course of optics it is known that a whole series of optical phenomena can be consistently described from a wave point of view; examples are the well-known phenomena of interference and diffraction of light. On the other hand (let us refer to the Compton effect discussed in the previous paragraph), light just as clearly demonstrates its corpuscular nature. This wave-particle dualism must be considered an experimental fact, and therefore a consistent theory of light must be a particle-wave theory. Of course, in some limiting cases, only wave or only corpuscular descriptions may be sufficient.
It turns out, and at the same time we will again refer to experiment, that particles of matter with non-zero mass (these include, for example, electrons, protons, neutrons, atoms, molecules, etc.) also exhibit wave properties, so between them and photons there is no fundamental difference.
At this point, when moving from macro to micro objects, a certain difficulty arises in understanding the essence of physical phenomena. Indeed, at the level of macrophenomena, the corpuscular and wave descriptions are clearly distinguished. At the level of micro-phenomena, this boundary is largely blurred and the movement of a micro-object becomes both wave and corpuscular. In other words, a situation in which a microobject is to some extent similar to a corpuscle, and to some extent to a wave, becomes more adequate to reality, and this measure depends on the physical conditions of observation of the microobject.
A consistent theory that takes into account this feature of all microparticles is quantum theory. But before moving on to the presentation of its main ideas, it is necessary to establish how one and the same physical object can, in principle, exhibit either corpuscular or wave properties and what comparability exists between these two different methods of description.
In optical phenomena, a criterion for the applicability of the concept of a ray (i.e., a corpuscular picture) has been established and rules for the transition from wave concepts to corpuscular ones have been found. Continuing reasoning in this direction, one can hope! that here lies the transition in the opposite direction: from the corpuscular concepts of classical mechanics to the wave concepts of quantum mechanics.
The corresponding ideas, using the optical-mechanical analogy, were expressed by the French physicist L. de Broglie in 1924. De Broglie put forward a bold hypothesis that the wave-particle duality is not a feature of optical phenomena alone, but has universal applicability throughout physics of the microworld. In his book "Revolution in Physics" he wrote: "In optics for a century the corpuscular method of consideration was too neglected in comparison with the wave method; was not the opposite error made in the theory of matter? Have we not thought too much about the picture of "particles" and neglected Is it too much of a picture of waves?”
The following considerations also led him to the assumption of wave properties in material particles. At the end of the 20s of the XIX century. V. Hamilton drew attention to the amazing analogy between geometric optics and classical (Newtonian) mechanics. It was shown that the basic laws of these branches of physics, which are so different at first glance, can be represented in a mathematically identical form. As a result, instead of considering the motion of a particle in an external field with potential energy, one can study the propagation of a light beam in an optically inhomogeneous medium with an appropriately selected refractive index. Of course, this equivalence of descriptions also allows for a reverse transition.
The noted analogy was extended by Hamilton only to geometric optics and classical mechanics. But, as already noted, geometric optics is an approximation of more general wave optics and does not describe the purely wave properties of light. In turn, classical mechanics also has a limited range of applicability: as is known, it cannot explain the existence of discrete energy levels in atomic systems.
De Broglie's idea was to extend the analogy between optics and mechanics and compare wave mechanics with wave optics, attempting to apply the latter to intra-atomic phenomena. “An attempt to attribute to the electron, and in general to all particles, like photons, a dual nature, to endow them with wave corpuscular properties interconnected by a quantum of action (Planck’s constant) - such a task seemed extremely necessary and fruitful... It is necessary to create a new mechanics of a wave nature, which will treat old mechanics as wave optics is to geometric optics,” wrote de Broglie in his book “Revolution in Physics.”
For the discovery of the wave properties of matter, L. de Broglie was awarded the Nobel Prize in 1929.
Let us now turn to the formal side of the issue. Let us have a microparticle (for example, an electron) with mass M moving in a vacuum at a constant speed. Using the corpuscular description, we attribute energy to the particle E and momentum in accordance with the formulas (consider the general case of a relativistic particle).
 . (1.2.1)
. (1.2.1)
On the other hand, in the wave picture we use the concepts of frequency and wavelength (or wave number). If both descriptions are different aspects of the same physical object, then there must be an unambiguous relationship between them. Following de Broglie, let us transfer to the case of particles of matter the same rules of transition from one picture to another, which are valid when applied to light:
![]() (1.2.2)
(1.2.2)
Relations (1.2.2) are called de Broglie formula. The wavelength associated with the particle is given by
![]() (1.2.3)
(1.2.3)
They call her De Broglie wavelength. It is not difficult to understand, by analogy with light, that it is precisely this wavelength that should appear in the criteria for the applicability of wave or corpuscular pictures.
The simplest type of wave in a vacuum with a certain frequency and wave vector is a plane monochromatic wave
A series of experiments carried out in the 10s - 20s. XX century, showed that particles, which were usually thought of as “building blocks of the universe”, solid balls – corpuscles, exhibit wave properties. Electron diffraction on a crystal has been demonstrated, i.e. the electron beam behaved similarly to an electromagnetic wave. In 1924, Louis de Broglie hypothesized that all particles (and therefore all bodies consisting of these particles) have wave properties. The measure of these wave properties is the so-called de Broglie wavelength . Indeed, let us compare a quantum (photon) of frequency n and wavelength l = c/n and an electron with momentum р = m e v:
![]() .
.
The value of l B for ordinary bodies is extremely small, and their wave properties cannot be observed (remember: for diffraction it was required that the size of the object be of the order of l). That is why the wave properties of only such light particles as an electron appear in experiment. The largest objects for which wave properties have been demonstrated are fullerene molecules C 60 and C 70 (mass ~ 10 -24 kg).
So , one of the most important concepts of our time is the idea of the unity of all forms of matter, substance, and field. There are no fundamental differences between them; matter can manifest itself both as a substance and as a field. This concept is called particle-wave dualism (duality) of matter.
At the same time, we are forced to characterize all observable quantities in terms of classical science, i.e. at the level of the macrocosm in which we ourselves exist. It is difficult for us to imagine an object that is both a particle and a wave, since we do not encounter such objects in everyday life. It is necessary to separate these concepts for methodological purposes. The reasons lie in the complexity of our structure as thinking beings. The science of cybernetics shows that a self-reproducing system must have a high level of complexity. We study the microworld as if from the outside, being structured immeasurably more complex than its objects. It is precisely and only for this reason that the dualism of matter does not seem to us an obvious, natural, inherent property of it.
3. Dynamics of microparticles. Heisenberg uncertainty principle
If a particle exhibits the properties of a wave, then it is as if blurred in space, representing a wave packet. In this case, it is impossible to talk about its coordinates. But is it not possible, for example, to take the beginning of a wave packet or the coordinate of the maximum of its envelope as such?
It turns out that the uncertainty of the coordinates of a microparticle is a fundamental property of the microworld; moreover, the speed of a microparticle also cannot be accurately measured. This fact has nothing to do with the accuracy of the measuring instruments.
Indeed, imagine that we are trying to measure the position and speed of a particle and use light for this. The minimum distance we can measure will be determined by the wavelength of this light, and the smaller it is, the more accurate the measurement will be. But the shorter the wavelength of light, the higher its frequency and the greater the energy of the quantum. A quantum with high energy will interact with the particle under study and transfer part of its energy to it. The speed that we ultimately measure will not be the desired initial speed of the particle, but a consequence of its interaction with the measuring device. So, the more accurately we measure the coordinate, the less accurate the speed measurement is, and vice versa.
For the wave x p = l E/c = l hn/c =l h/l = h– this is maximum accuracy.
Formula expressing the relationship between uncertainties in finding a coordinate X and momentum r particles, was first obtained by W. Heisenberg and bears his name:
Dх Dр ³ h –
- Heisenberg uncertainty principle.
Similar relationships hold for the uncertainties Dу and Dz.
For energy and time uncertainties we obtain:
So, the uncertainty principle is a fundamental property of nature, in no way connected with the imperfection of measuring instruments, but of a fundamental nature.
The principle of uncertainty, along with the concept of quanta, formed the basis of the new quantum mechanics, the ideas and range of problems of which were revolutionary in a way different from everything previously known to science. The scientific paradigm was broken, a fundamentally new approach to considering the phenomena of the microworld arose, which later turned out to be very fruitful in other areas of science.